Okidokes. So as many of my readers should know by now, I have a B.S. in Physics. I also happen to be very skeptical when it comes to sensationalist claims. Usually these take the form of some ‘Green initiative’. The goal being to appeal using virtue signal (saving the planet) while also claiming to be cheaper (economic incentive). Solar roadways were a bust. The Fontus water bottle was also a bust. But they still gathered a lot of traction due to being green and claiming to be cheaper and better for the world. And this hearkens back to the post I made about the lottery… what? I didn’t make a post about it? Maybe it was only a Facebook rant. Anyway there was a post a while ago saying that we could solve poverty by splitting the lottery jackpot among all United States citizens. They claimed we could give everyone about $4 million dollars!

I don’t know whether @Livesosa or Philipe Andolini came up with this. However, the math is completely wrong. Everyone would get about $4. Enough to buy everyone a small pocket calculator to check their math. The worst part is that I tried to justify this by remembering that we in the states (and eventually the entire world) use short form when it comes to large numbers. What this means, for those of you not in the know, is that when we say ‘a billion’ what is understood is that we are referring to 1000 millions. In long form, ‘a billion’ would refer to a million millions. The problem is that the order of magnitude is off by a thousand, so I could not justify the calculation. In other words, people are just dumb.
Anyway, so I came across this new type of air conditioner. It’s called Geizeer and the hyperlink leads to their kickstarter. I plan to take on their claims and apply physics to evaluate their truth values. By the end of this post, we might be able to determine whether or not donating to it is a waste of money. So what are the claims?
CLAIM #1
The first claim is that you will be able to cool your home by 3° C for a (well-insulated) 12 square meter room. So first, we need to think about how large 12 square meters is. And this is where I often run into issues of convention. By my interpretation, 12 square meters would be the area covered by a rectangle that has sides of length three meters and four meters. So to put it into terms we Americans are familiar with, it’s about a 10′ x 13′ rectangle. Which is probably close to the size of my apartment’s floor now that I look around. So it’s not what I would call a small area. Because you want the room to be cool, not just a plane, let’s tack on a third dimension. Realistically you’re going to have an expanding ‘sphere of cooling’ because of the way temperature diffusion works. So our third dimension should be about the height of a room. The height of a room varies based on… well, the room really. So I’m going to stand up in my room and just guesstimate the height. [Will the real Artemis Hunt please stand up] Okay, looks like it’s probably close to nine feet tall. So that’s about 2.75 meters. So the dimensions of the final room which we will be considering for the sake of this thought experiment and calculation is 3m x 4m x 2.75m.
It also claims to cool the air by 3° C for this area. How large of a temperature change is that? Close to about 6° F. This is not a significant change. Room temperature is usually defined to be about 20° C or 21° C. This is close to about 68° F or 70° F. Just for the sake of consistency and to not have to do a ton of range calculations, let’s just stick to 21° C and 70° F being room temperature. The effective change in temperature would bring the room down to 64° F. But you’re likely not using air conditioner if the room is at room temperature. No, you’re probably using it to keep the house AT 70°. Which means your room is around 76° F.
All of this temperature stuff was just to give you an idea of the scale of changes going on. The calculations I’ll be performing are to lower a room from 24° C to 21° C. The first thing we need to do is figure out what equations we’re going to use. Perhaps you’re familiar with an old post of mine in which we did similar calculations, applying it to cartoon physics. We’re going to be using the same equations we used from that post, just with different things. So what equation do we need? Good old rapper name mcDeltaT
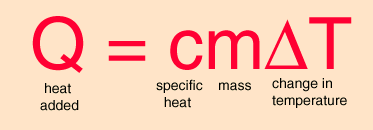
We want to figure out how much heat is necessary to change the temperature of a cube of air from one temperature to another. Now you’ll note that the equation refers to heat added. Initially you might be inclined to think that the equation is not applicable. After all, it says heat ADDED. However we want to cool the air! Surely we wouldn’t want to add heat to cool the air, right? Well, fret not, because the equation works both ways. Remember that Δ generally refers to the difference of a final condition and an initial condition. And I worded it that way intentionally; it has to be final condition minus initial condition. Subtraction is not commutative. The order in which you subtract things matters. And if you don’t believe me you can test it yourself. Subtract three from four and four from three and tell me whether or not you get the same answer both times.
Why am I emphasizing that the difference must be final condition minus initial condition? Well, let’s do a small thought experiment to demonstrate the concept. If the final temperature is higher than the initial temperature, then the ΔT will have a positive value. If the final temperature is lower than the initial temperature, then the ΔT will have a negative value. Mass m will obviously always be positive and specific heat c is defined to be positive. Just as a reminder, specific heat is the a measure of how much energy must be dumped into a mass to raise it by one degree Celsius.
So the only thing that can change the sign of Q, our heat added, is the sign of ΔT. If the temperature increases, ΔT is positive meaning heat was added to the system. If ΔT is negative, that means heat was removed from the system. So the equation will still work just fine, we will just have a minus sign to denote that heat was removed from the system.
So now we need to figure out what c and m are for the air. We know ΔT already (24° C – 21° C = 3° C). According to this source, the specific heat of at around room temperature is about 1.005 kJ/kg*K. So we’ll roll with that. What about the mass of the air? This has to be horrendously tiny. Well… the density of air depends on on its temperature and pressure. We’ll be assuming standard atmospheric pressure because that’s what most rooms you might be using this in will be at. We’ll also use the density at room temperature because it’s reasonably close to that. We’ll be reducing the temperature by about 12.5 percent. Also since we’re dealing with a closed system, we shouldn’t expect the volume (and by extension, mass and density) to change. So from the same page, we see that the density of air at room temperature and standard atmospheric pressure to be 1.205 kg/m^3. Now if we include the temperature change, we can see how much energy we need to remove from the system to produce the temperature change.

Holy smokes, are there really 39 kg of air in my room? Probably not. Myself and my furniture displace a lot of the air so realistically the number is not quite as large. But 39 kg? That’s amazing! I wasn’t expecting such a large number, huh. Anyway, let’s carry on. So that’s the mass of the air in my room in kilograms. Let’s complete the equation to see how much energy we must add to the system to cool it by 3 degrees Celsius.
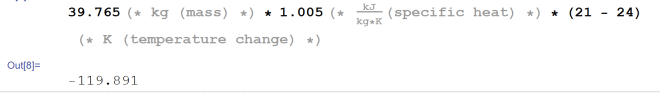
You may have noticed that I put the units of temperature change in Kelvin, rather than Celsius. Surely that would be breaking the rules? No, it’s not. Well, not in the end. The Kelvin scale is a rescaling of the Celsius scale. The only difference is where the zero is placed. So a change in a degree Kelvin is a change in a degree Celsius. At the end of the day, we see that we need to add -119.891 kJ of energy to the air in the room to produce this temperature change. If it makes you feel better, you may say the same thing by saying we need to remove 119.891 kJ of energy from the air in the room to produce this temperature change.
Now we get into one of the most important laws of physics. Its use has guided our hand in thermodynamics through the centuries. It is practically one of our ten commandments. What I am referring to is the Law of Conservation of Energy. We want to remove energy from the air in the room. But we’re not going to dump it out of the room. Remember that qualifier in the claim. The room has to be well-insulated. This means that energy doesn’t enter the room, and energy doesn’t leave the room. The room is a self-contained environment. What does this mean for the energy? Well it can’t leave the room, so it has to go somewhere else in the room. The natural flow of heat is from higher temperature regions to low temperature regions. Now the Geizeer actually provided such a low temperature region. There’s some kind of gel pack in its center which has been frozen in the refrigerator. Its temperature would probably be comparable to that of something in your freezer. Heat flows from higher temperature regions to lower temperature regions. So the heat in the warm air will flow to this cool gel pack. As the energy leaves the air and is absorbed by the gel pack, the air cools down (and the gel pack heats up).
Unfortunately it becomes very difficult to evaluate the energy necessary to change the temperature of ice packs without knowing what they’re made of. If it’s ice, we’re good to go. I can easily evaluate the physical properties of ice. But it’s some kind of weird gel that I can’t get the material properties of, then my journey stops here. So let’s just look at the ice situation. I would imagine that if ice were better, it would be used instead of some gel. Therefore ice is probably the worse of the two materials to use for the ice pack. So if our calculations work for ice, then we can be reasonably sure that they would work for the gel. However if the calculations don’t work for ice, we may be stuck with no way to decisively conclude anything.
So we’re going to use that heat added equation. But we’re going to rearrange it to solve for mass. We know the specific heat of ice. By this source it’s… temperature dependent. Ouch. Well let’s at least figure out how cold it would be at the outset. If we assume that the inside of the freezer is maintained at a temperature, we can assume that when we remove the ice pack that it will be at that temperature. So what temperature would that be? Well, according to the USDA it should be at 0° F. Which is about -17.8° C. So when we remove the ice pack from the freezer it will be about -17.8° C as well. As the ice pack increases in temperature, its specific heat changes. So let’s use an average specific heat over the range of ice temperatures. The average specific heat from -20° C to 0° C is 1.9984 kJ/kg*K. So that is the value that I will use. Since the density also varies by temperature and we calculated an average specific heat, we should probably do an average density as well. In that case, the average density of our ice will be 918.28 kg/m^3
Now we need to figure out the mass of the ice. Granted, the entire block is not made of ice but we will assume it is for the sake of simplicity. But how are we going to do that? This is where I start doing some pixel math. Let’s use this image
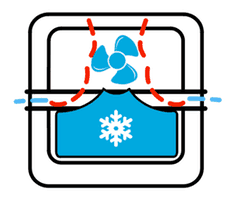
Assuming cubical symmetry we can probably figure everything out. So the box itself is 175 pixels tall. The kickstarter page lists the height of the box to be 134 mm. The length of the box is stated to be 144 mm. So let’s check the length to see if we get a comparable number. Doing another measurement I see that the box is 175 px by 175 px. So they have it as a square. What does this mean for our calculations? It means that the picture is likely not drawn to scale. If it is drawn to scale, then it means the resolution of each pixel probably has an uncertainty of 5 mm. But I’m too far in to pull out now, so forge ahead we must. Since it’s a square, let’s just take the average of the two lengths, or 139 mm and say that 175 pixels is 139 mm. Now let’s get the dimensions of the ice pack. And this is where we run into a bit of a problem. It’s not a simple object like a cube. So to get the volume of the ice pack, I’ll assume it is two separate objects. A rectangular prism on top of a rectangular prism.
First, let’s look at the bottom rectangular prism. Again, we’ll be assuming cubical symmetry, so its length will also be its width. And that length happens to be 120 pixels. Using our scale of 175 pixels being 139 mm, we conclude that 120 pixels is 95.314 mm. Now let’s get the height of the rectangular prism. Counting pixels, we see that it’s 44 pixels tall, or 34.949 mm. So the dimensions of the rectangular prism below are 95.314 mm x 95.314 mm x 34.949 mm. Now let’s attempt to approximate the cube on top. Now I’m going to display to you the box that I will be approximating our smaller rectangular prism with.
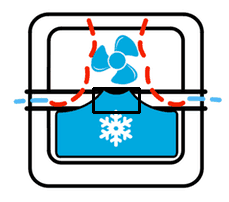
Yes, some of the sloping sides are not included but the middle part isn’t entirely filled so this eyeballing should be good enough to give us some ballpark results. The length is 45 pixels and the height is 25 pixels. Wow, I free-handed that box, and got such nice numbers? Let’s convert those pixels to real-world measurements. Converting the pixel measurements, we get a length of 35.743 mm and a height of 19.857 mm. So the dimensions of the smaller prism are 35.743 mm x 35.743 mm x 19.857 mm.
Now we have the dimensions of the boxes. And it is here that we will convert our measurements to meters. Why? Because we have the density of ice in kg/m^3 and I don’t want to deal with converting cubic millimeters to cubic meters. Much better to start with cubic meters. So let’s do that.
Big Prism: 95.314 mm x 95.314 mm x 34.949 mm = 0.095314 m x 0.095314 m x 0.034949 m
Lil Prism: 35.743 mm x 35.743 mm x 19.857 mm = 0.035743 m x 0.035743 m x 0.019857 m
(Oh wow, I never noticed that the font wasn’t monospaced. I’m too used to programming)
We’re almost ready to use our heat equation. Now we just need to get the volume of our sum prisms. This happens to be 0.000342872 m^3. To get the mass of this ice, we multiply the volume we found ( 0.000342872 m^3 ) by the density we settled on earlier (918.28 kg/m^3) and what we get is a block of ice with a mass of 0.3149 kg. Before we go any further, let’s do a little bit of a check. Does this seem reasonable? Well, that’s about .7 lbs. It weighs about as much as a baseball. That seems about right for an ice pack. So our guess doesn’t seem unreasonable.
Alright! So we have our mass (0.3149 kg), we have our specific heat (1.9984 kJ/kg*K), all we need now is our change in temperature! If we permit the ice block to come into thermal equilibrium with the room, we will see a change in temperature of 39° C. Final temperature (21° C) minus initial temperature (-18° C). I personally do not think that this would be the actual change in temperature, but we may as well do the calculation to see what would happen as time went to infinity. We do need to note a few things though. First, ice won’t exist above 0° C. Water will. So we can only do the heat equation for the energy necessary to raise the temperature of ice from -18° C to 0° C and the energy necessary to raise the temperature of water from 0° C to 21° C. We’ll have to use a different equation for the melting bit but that’s simple enough. It’s the mass of the object multiplied by its latent heat of fusion. The latent heat of fusion of ice/water being 334 kJ/kg. So here’s the process step by step.
- Calculate the energy required to raise the temperature of ice to 0° C
- Calculate the energy required to melt all of that ice into water
- Calculate the energy required to raise the temperature of water to 21° C
Simple enough, right? Let’s get at it. First, we need the energy required to raise the temperature of ice to 0° C from -18° C.
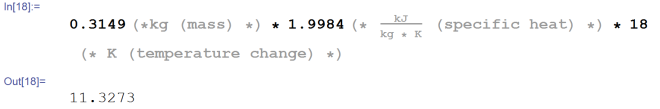
11.3273 kJ. Alright, now let’s figure out how much energy we need to melt all of the ice
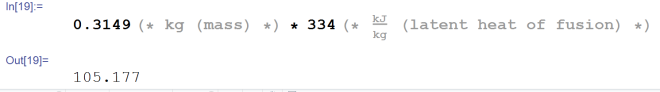
105.177 kJ. Excellent, moving right along. Now the ice has been fully melted into water at 0° C. We didn’t add any mass, we didn’t take any away. it was only a phase change. The only thing that really changed was the density of water (it increased). The specific heat of water however, is very different from that of ice. The specific heat of water is 4.186 kj/kg*K. That’s one you memorize quite quickly whenever you do enough of these types of problems. Here’s the energy calculation for the water.
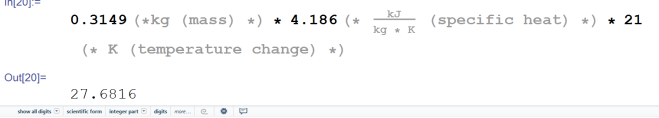
Alright, now for the final step, let’s add all of these energies together. That’s 11.3273 kJ + 105.177 kJ + 27.6816 kJ = 144.1859 kJ. That’s how much energy that must be dumped into the ice pack to produce that type of temperature change. Now the question is… do we have enough? Remember how much energy the air needed to lose to drop 3° C? It was 119.891 kJ. We have 144 kJ of energy. So we do have enough… if we raise the temperature of the ice pack from -18° C to water at 21° C. Let’s figure out how high the temperature of the ice pack has to become to cool the room exactly. To do that, we first subtract the energy necessary to raise the ice to melting temperature and the energy necessary to melt the ice from the amount we predicted would be necessary to lower the air’s temperature. So 119.891 kJ – 11.3273 kJ – 105.177 kJ = 3.3867 kJ. Now we solve for change in temperature using 3.3867 as our heat added value. We get this:
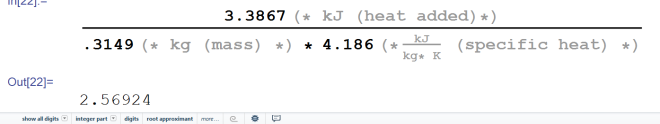
The water would have to be raised to a little over 2.5° C for this to work.
So what does this all mean? It means that in this worst case check, for which the ice pack is literally made of ice, that there is a place to put the energy we removed from the air to cool the room. In fact, we could even cool it a little bit more. Especially when you remember that we likely overestimated the mass of the air in the room because your room probably has furniture in it – which take of space that air could be taking up. Of course… that presents its own slew of problems which we will not be addressing. This blog post is long enough already and I still have another claim to address! So this part of the Geizeer gets a pass by my calculations.
CLAIM #2
In fact, using it for the duration of 24 hours will have an economic consumption of less than 1 cent.
Let’s check that one out. So the device facilitates the transfer of energy through the use of a fan. This fan is powered by a 3.7 V battery with a lifetime of 7 hours. This means that you must recharge the battery at least twice if you want to run the device for 24 hours. So it’s theoretically impossible to have it running all day however that doesn’t mean we can’t evaluate the claim of energy cost. The fact that I have no idea how much energy is required to charge a battery would mean we can’t evaluate the claim of energy cost. Maybe we can get around that though.
What do we know? We know the energy necessary to to cool the air as claimed. We know that each of these ice packs supposedly works for four hours. This allows us to figure out how much energy is being dumped into the ice pack(s) per day. This next calculation works regardless of whether or not the ice pack is ice or some kind of gel. The reason being that we’re calculating how much energy is being dumped into it per day, not whether or not there’s enough energy for the temperature change to occur. We calculated earlier that we need to remove 119.891 kJ of energy from the air. This is the work necessary to cool the air. We do this over four hours. This means we’re operating at about 8.3258 Watts (8.3258 Joules per second). Why have I done this conversion? Because the cost of power in the United States is rated by kW*hr. The average being about 12 cents per kiloWatt hour. So if we want to figure out how much this power would cost, we multiply by the kiloWatts we’re using and by 24 hours because we’re doing this by the day. We’re using 0.0083258 kW and we’re doing it for 24 hours, so the cost of the energy transfer alone is…
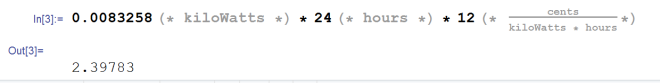
The claim is false. If you ran it all day it would cost you a little over 2 cents. However that’s just the cost to move the energy from one place to another. It does not include the cost necessary to operate your freezer to freeze the ice packs repeatedly. It doesn’t include the cost to operate the fan. Realistically, it will cost you far more than 2 cents per day. But not all of that cost is going directly to the Geizeer’s operation. Your freezer was likely already running all day long anyway, so the addition of the ice pack probably doesn’t add an effective cost to your home.
Now we come up to the big question. Is it cheaper to run a Geizeer instead of your usual air conditioning unit? That’s a hard claim for me to work with and let me begin by stating that I AM NOT AN EXPERT. If your home is controlled by some central air system like mine is, it’s working for far more than just one room. It’s working for your kitchen, your bedroom, your dining room, your living room, and your guest room. That’s a whole lot more air than this little apartment room that I calculated the numbers for. Sure, it’s 2.5 cents for one small room, but when you add up all of the rooms, do the numbers still work out? I can’t say for sure. I did look up a site to figure out how to compare the cost. But the problem is it just depends on the size of the house and the type of air conditioner far too much. But their example is well over even $1 which I don’t see a number of these Geizeers in your home ever reaching. one thing to note, is that you need to buy the Geizeers, while you likely just pay for the operation of your current air conditioner. So if you buy a Geizeer, you’ll likely recoup the cost of the Geizeer at a rate of one Geizeer per month. So at the end of the day, looks like the Geizeer passes my test. Despite one of its claims being false, it does seem to be effectively cheaper to operate compared to an air conditioner. It’s also portable, while your window AC or your house AC probably isn’t. (Shower thought: Do people even use window ACs anymore? I haven’t seen them in forever… but I do currently live in Alaska)
So the Geizeer gets a pass! Thumbsup.jpg
Here is the link to their Kickstarter again.
Artemis Hunt
[…] vsebine: geizeer.com /www.indiegogo.com / homeofthehuntress.wordpress.com Objavljeno: 25.08.2017 @ […]
LikeLike
“We calculated earlier that we need to remove 119.891 kJ of energy from the air. This is the work necessary to cool the air. We do this over four hours. This means we’re operating at about 8.3258 Watts (8.3258 Joules per second).” isn’t that 119891 J a needed cooling power? Maybe Italians measured consumpition for 4h when was achieved 3 degree of Celsius lower temperature and multiplied it by 6 to get all day actual consumption. Sometimes things in theory and practice are quite different because of false foresight. 8.3 W (J/s) was calculated from 4 hours (119891 divided by 4 hours and 3600 seconds per hour) and then multiplied by 24 hours to get watts in all day. Is Watt-second same as Watt-hour? How could be 119891 J transfered to watt power needed to cool it? Calculation of cooling power in Watts; 29,972.75 Watts on hour basis what is too big number or 8,3 W * 4h = 33,2 Wh / 199,2 W/24h.
I’m building my own Geizeer called Gajzer (slovenian translated term; we have also surname Gajzer that origins from germans). Italians in video pronounced it as Gajzer but in English it should be pronounced as Gajzir. I haven’t found from where that name. I have first thought that name comes from Geyser ( I haven’t know english name before; Slovenians call it gejzir) what is ridiculous because is it actually hot spring. But later I have found that in german Geiz means stinginess (little music for little money). My cooler works on same principle and has design but bigger size of housing (250x250x190mm), fan (200mm) and ice-pack (200x170x30mm) for cooling bags while diffuser is 3d printed to sit on top of ice-pack. I assume that it will consume maximum 5 € cents per day with 12V LiPo battery 9800mAh but I haven’t tested in practice, it’s still in building phase (wooden housing). Only difference will be in addition of silicagel cap from effervescent tablets as humidity remover and markers for right position to power on as well a zeolite powder ZC20 in tea bags below in compartment besides battery as a odor and humidity remover.
LikeLiked by 1 person